
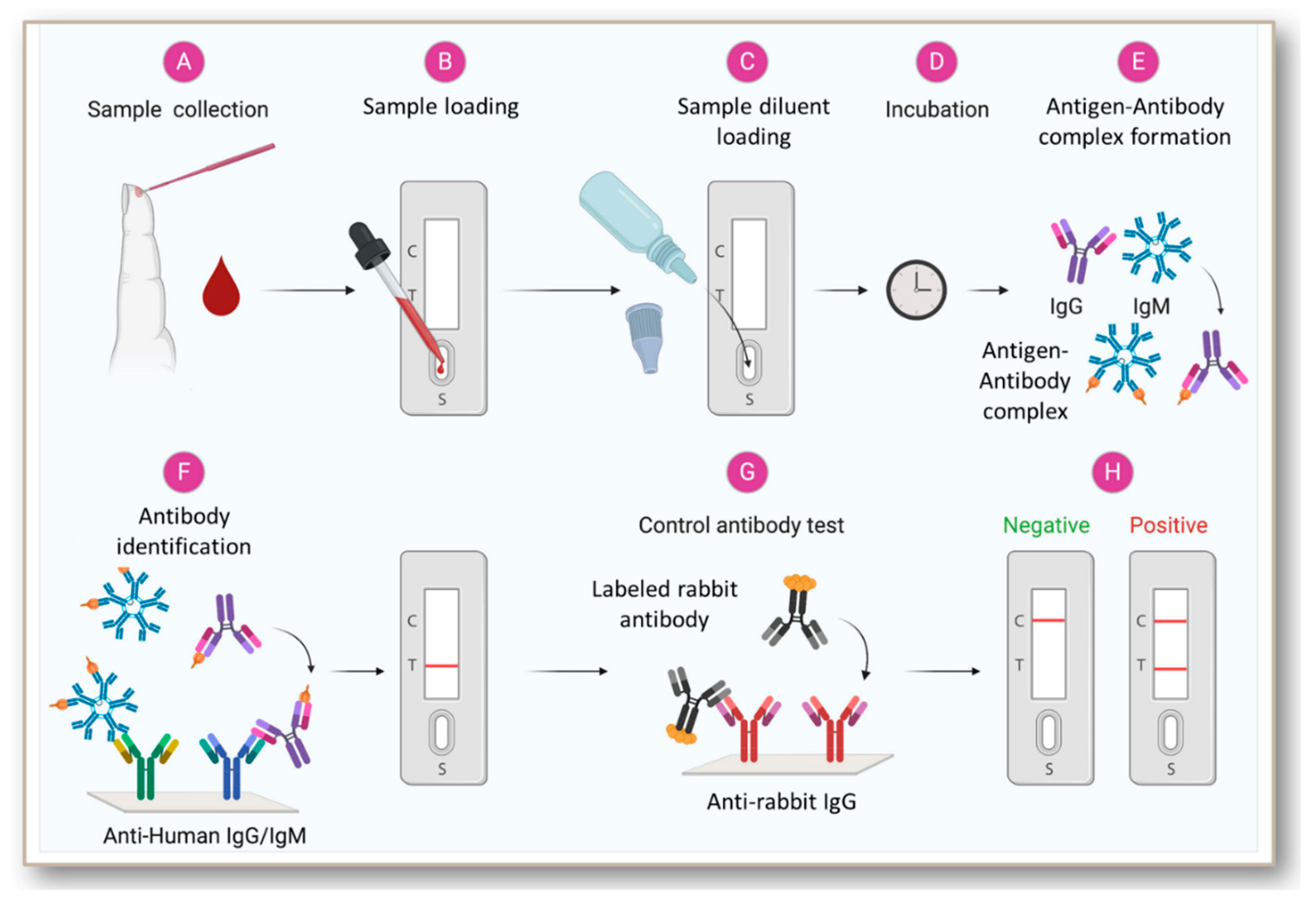
It sets out the clinical requirements based on the consensus of what is ‘minimally acceptable’ in the opinion of UK IVD industry, healthcare professionals and medical device regulators given the emergency situation. This is a specification of the clinically acceptable specifications for a laboratory Enzyme Immunoassay (EIA) test to be made and used in the UK during the current COVID-19 pandemic caused by SARS-CoV-2 virus. Some people may not develop an antibody response. A negative test does not guarantee no prior COVID-19 infection. Such tests usually require taking a blood sample and looking for the presence of antibodies specific to SARS-CoV-2, the causative agent of COVID-19.Ī positive result from this test does not guarantee immunity to COVID-19 infection and may not indicate the infectious status of the person. Implementation of Pillar 3 of the testing strategy relies on availability of antibody tests that could tell people whether they have had the virus. Production lead time will also factor into decision making. Anyĭeviation from existing standards must be fully justified. The TPP assists the UK government in making decisions regarding central procurement of antibody tests and might also be used in local procurement decisions. These product profiles have been developed to assist manufacturers to design and deliver tests that might be useful in support of Pillar 3 of the UK testing strategy. (2) the minimally acceptable profiles based on the intended use, setting of use, and intended user, with respect to the performance and operational characteristics expected of the target products. A TPP provides a common foundation for the development of tests that contains sufficient detail to allow device developers and key stakeholders to understand the characteristics a test must have to be successful for the particular intended use. They help guide industry development towardsĭesired characteristics. TPPs state intended use, target populations and other desired attributes of products, including safety and performance-related characteristics. Target product profiles ( TPP) outline the desired ‘profile’ or characteristics of a target product that is aimed at a particular disease or diseases. Target Product Profile 1.1 Enzyme Immunoassay (EIA) Antibody tests to help determine if people have antibodies to SARS-CoV-2ġ.3 The purpose of a Target Product Profile “ TPP”


 0 kommentar(er)
0 kommentar(er)
